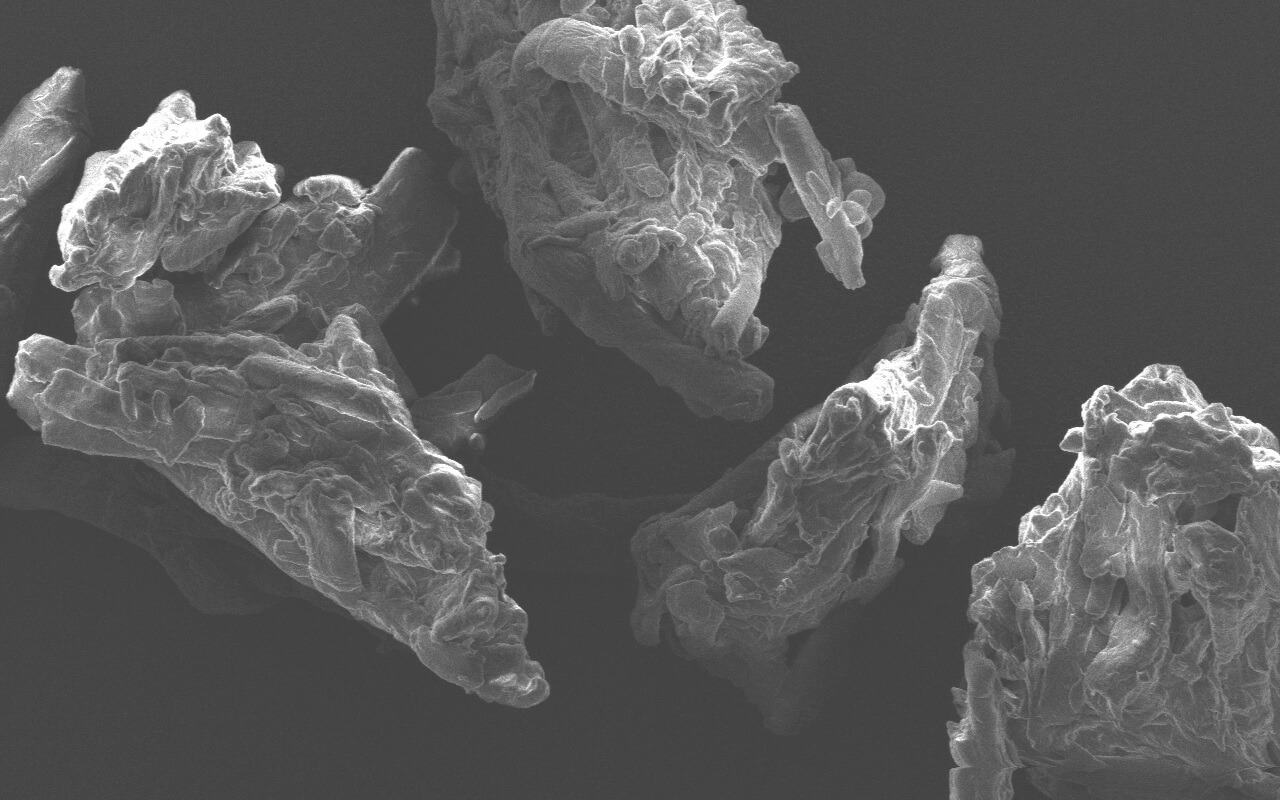
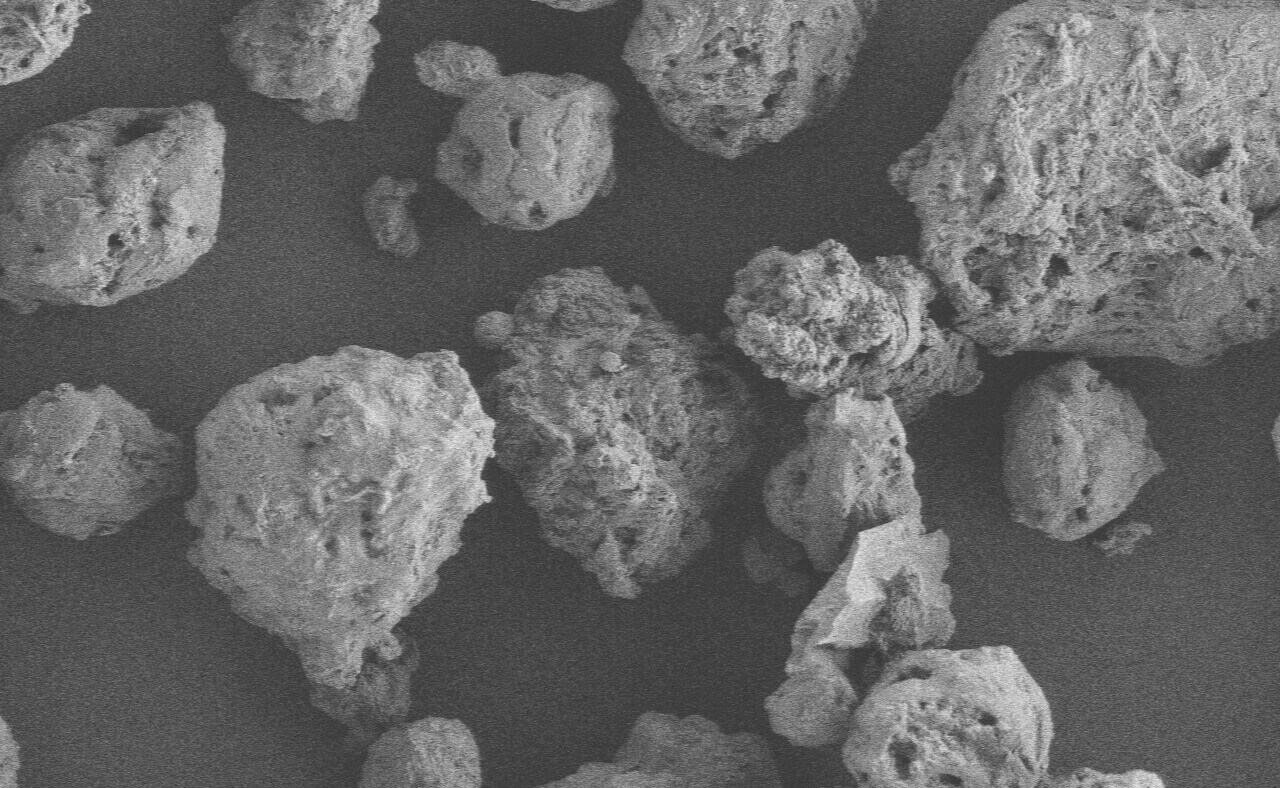
Products
MORE
Product Application

MCC difficult to choose? Not at all!

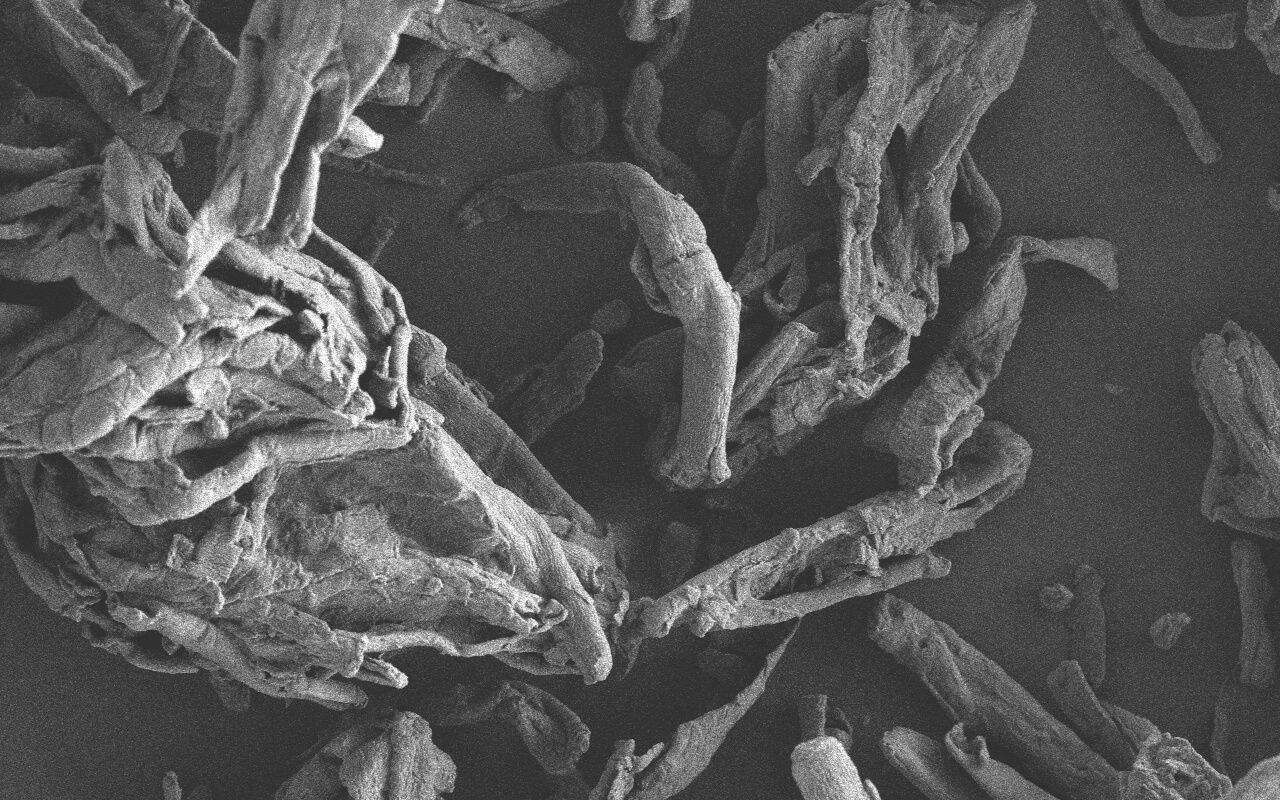
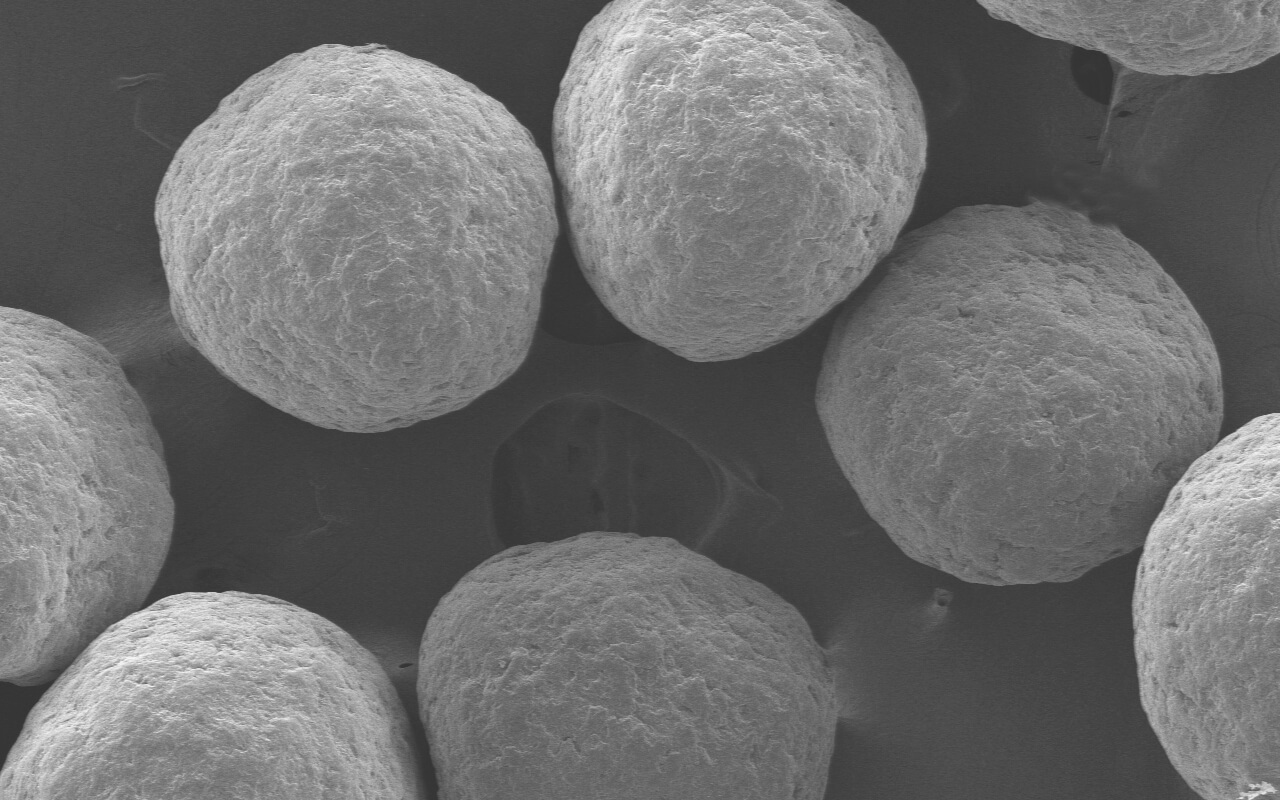
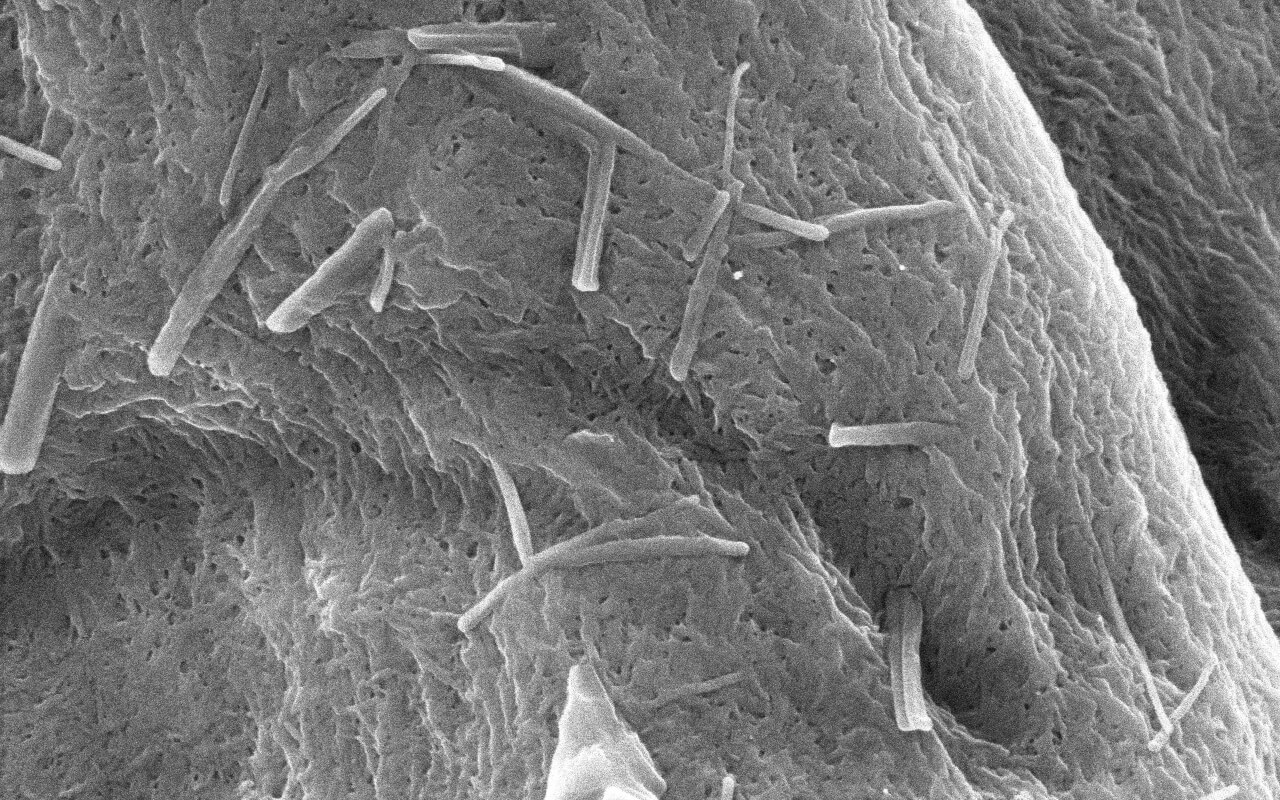
Research on Functionality of Co-processed Excipient TOMITOLTM TMT (TMT934)

Research on the Hygroscopicity of MCC sand MCC Co-processed Excipients
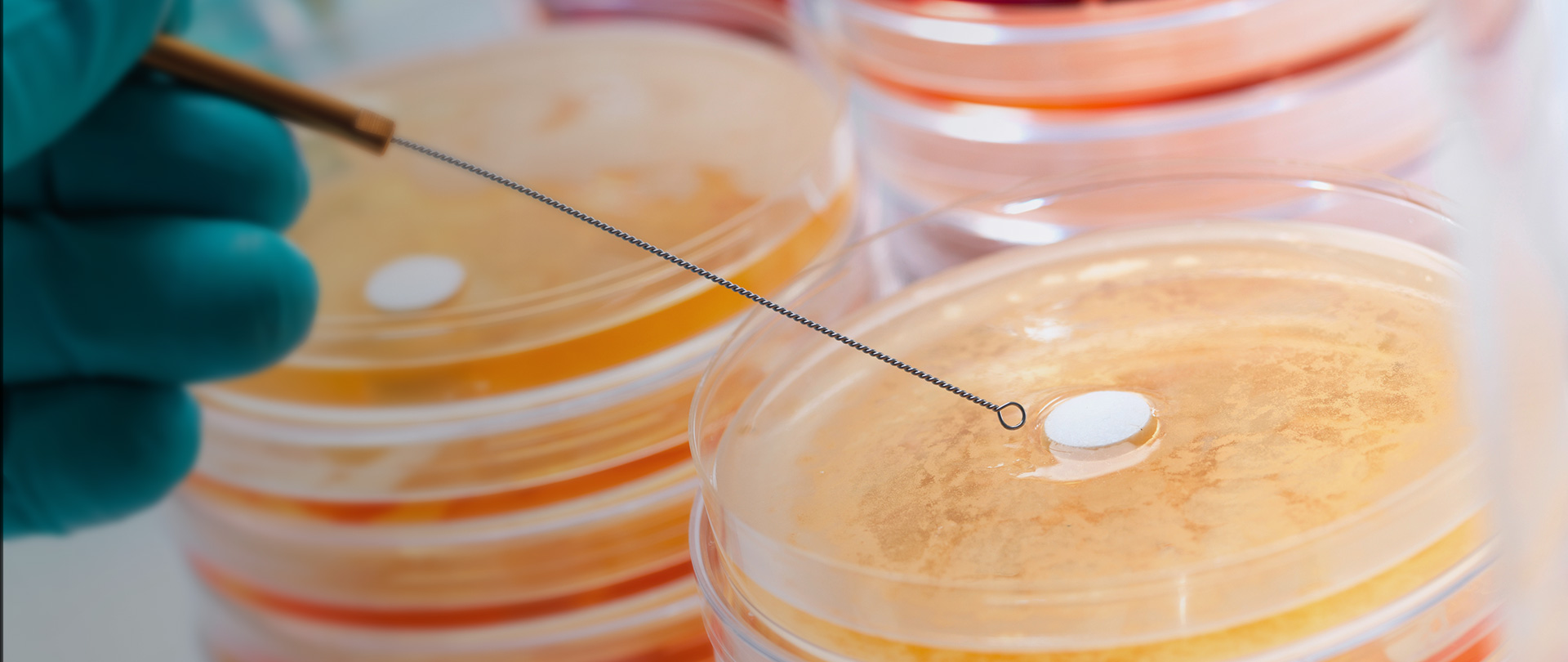
Microcrystalline cellulose (MCC) is widely used in tablet and capsule formulations, usually as a diluent or blinder. In addition, due to its certain lubricant and disintegrant properties, MCC is generally used as an adsorbent, suspending agent, tablet and capsule diluent and disintegrant in pharmaceutical preparations. It's suitable not only for wet granulation but also for direct compression processes. Now, there are many brands and models of MMC in the domestic market, either imported or domestically manufactured. At first glance, there are many choices, but this makes it more difficult to choose and inevitably fall into a dilemma of choice. For instance, choosing imported MCC may need to consider the prices, delivery period, supply stability, tariffs, compliance with regulations, etc.; while choosing domestically manufactured MCC may need to weigh the impact on consistency evaluation, quality stability, compatibility with imported models, risk of extended inspections required by regulations, etc. If there is a domestic brand whose products are comparable with or superior to imported ones in quality and complete in model and can be tailor made and produced by batches according to consumer needs;
MORE
As the level of pharmaceutical preparations continues to improve at home and abroad in recent years, more and more new pharmaceutical excipients have been developed and applied. Mixing existing excipients using an appropriate process and then applying to preparations as a whole, which maintains the chemical properties of each excipient without changing its safety and makes the original excipients have new functions, has become a new hotspot in the development of the international pharmaceutical excipient industry. Such excipients are called co-processed excipients or pre-mixed excipients. IPEC-Americas and IPEC Europe jointly released the co-processed excipient guide in 2017, which has further encouraged and stimulated their development.
MORE
There is no official method individually applicable to studying the hygroscopicity of excipients. Here, the research has been carried out with reference to the guidelines for the testing of drugs for hygroscopicity in Volume IV of Chinese Pharmacopoeia 2015, which describes the concept of drug hygroscopicity and defines the hygroscopic weight gain as follows:
MORE
MORE
MORE
MORE
-
2012yearTime of establishment
-
20 +peopleScientific researchers
-
20 +Qualification certificate
-
1000 +㎡Class D workshop
-
8000 +㎡Existing plant
News


 En
En